Max Planck Emeritus Group
Rhythms - Beating Cilia and Ticking Clocks
(Prof. Dr. Gregor Eichele)
The brain is a compact mass of neurons forming networks that define who we are and direct what we do. Inside this brain machine is a branched system of ventricles – cavities - through which flows cerebrospinal fluid (CSF), a substance-rich liquor. Greek and medieval physicians regarded the CSF filled ventricles as the seat of thinking, memory, consciousness and feelings. Medical science later degraded ventricles to a drainage system filled with compounds that the brain needs to dispose of. Research during the past decades found that CSF contains neuroactive signaling molecules and not just waste. Thus the interconnected ventricles emerge as canals that transport and deliver signaling factors to specific brain regions.
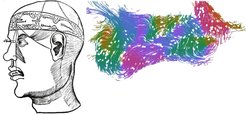
To execute this transport function, the walls of the ventricle carry precisely positioned motile cilia that set in motion and guide CSF flows over distances of millimeters and even centimeters. The cilium is eyelash-shaped and protrudes from the cell surface. Inside the cell, the cilium is attached to a basal body. In the cells of the brain ventricles, cilia form bundles made of 30-60 units and each cilium is equipped with motility proteins that make cilia beat in a whip-like fashion. In effect, this cilia whipping pushes CSF along the ventricular wall in diverse but precisely defined directions.
In recent years, we have studied cilia-driven transport in the brain ventricles (doi.org/10.1126/science.aae0450). We focus on the third ventricle that resides in the hypothalamus, a brain region that regulates many endocrine functions in the body. We investigate when and how during brain development directional CSF flows are established. A key process of directional flow development is imposing asymmetry to the cells that form the ventricular wall. Therefore, we study when cell asymmetry first appears, and how the spatiotemporal expression pattern of plasma membrane-associated cell polarity proteins confer cell polarity and cilia beating direction.
that form a grid-like array that gets its geometry from polarity-conferring transmembrane proteins (right).
We investigate the geometry of transport routes within the third ventricle by locally applying fluorescent liposomes and video-recording vesicle transport. This led to the discovery of cilia-driven micro-flows, only a few cells wide, “crawling” along the walls of the ventricle and thereby delivering vesicle content - cargo - to target regions. The microflows obey a set of traffic rules that allow a well-ordered distribution of cargo. More recently, our studies include the transport of extracellular vesicles (EVs) that naturally occur in CSF.
We also investigate which signaling factors are contained in extracellular vesicles. EVs are produced within the ventricular cavities by a secretory epithelium termed the choroid plexus. We isolate the vesicles from choroid plexus cells and study how purified vesicles regulate the physiology and behavior of stem cells that reside in a particular sub region of the third ventricle. Methods of study include functional analysis of stem cells that differentiate upon contact with our purified EVs.
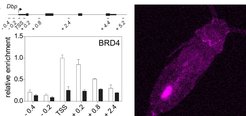
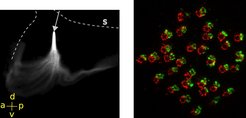
BMAL1 proteins must bind - in a 24-h-rhythm - near the transcription start site (TSS) of clock-controlled genes such as Dbp (left). To track circadian clock-controlled locomotion as is occurring in DVM - over days - this Arcatia tonsa copepode was fed with fluorescent algae that form a trackable, fluorescent dot in the intestine (right).
We carry out protein mass spectrometric analyses of both, the EVs and the recipient stem cells. The aim is to identify molecules that the microflows transport and eventually deliver to the stem cells. The broader significance of this work is that it investigates a cilia-driven, directional transport system that delivers signaling factors within ventricles and to brain tissue underneath the ventricular wall. The transport is powerful enough to directionally move nanoscopic particles within seconds and do so in a low Reynolds number environment.
A long standing interest of Eichele is the molecular clockwork that drives many physiological and behavioral processes (doi.org/10.7554/eLife.43235) including dial vertical migration (DVM) of plankton. DVM is colossal, daily translocation of biomass and profoundly influences the food chain in the world’s oceans. In collaboration with Dr. J. Söding (MPIBPC) and Prof. em. O. Larink (University of Braunschweig) we determine the transcriptome sequence of a variety of zooplankton species with the focus on their circadian clock system and the role of this clock in regulating the oceanic DVM. This research encompasses laboratory cultured plankton and fresh plankton collected at the AWI Helgoland and in the Atlantic Ocean on board of S/Y Eugen Seibold (MPIC, Mainz).
Image credits: S. Kapoor, A.-K. Günther, C. Westendorf, N. Petkau


