
Surfactant-laden Liquid Crystal Interfaces
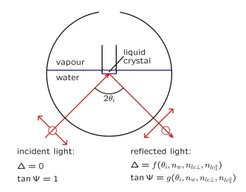
Fig. 1: Schematic experimental setup for ellipsometric studies of liquid-crystal/water interfaces. A laser beam is reflected by the interface and the corresponding change of the state of polarization, described by the two parameters Δ and Ψ, is detected. The results yield information about the refractive index profile of the interface.

Fig. 2: Temperature dependence of the ellipticity coefficient ρ at surfactant-laden 8CB/water interfaces in the vicinity of the nematic − isotropic bulk transition temperature Tb. The concentration of the surfactant CTAB in the aqueous phase amounts to 0.8 μM, 0.7 μM, 0.6 μM, and 0.5 μM (from top to bottom). Small blue dots are experimental values, black open circles correspond to calculated values resulting from a Landau-de Gennes model, insets show the temperature dependence of the Brewster angle θB. Whereas the data at higher surfactant concentration (top figure) show a complete wetting by a more ordered (nematic) phase as Tb is approached from above, the data at low surfactant concentration (bottom figure) indicate that the bulk nematic/water interface is wetted by a less ordered (nearly isotropic) phase as Tb is approached from below.
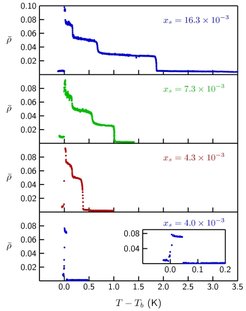
Fig. 3: Ellipticity coefficient ρ as a function of the temperature difference to the smectic-A − isotropic bulk transition temperature Tb at 12CB/water interfaces. The 12CB volume phase is doped with a small amount of the nonionic surfactant monoolein, the values xs give the mol fraction of the surfactant in the bulk liquid-crystal phase. The data for xs = 0.0043 indicate a 0↔2-layer transition and for xs = 0.0040 a 0↔3-layer transition (the inset displays the same data on an expanded temperature scale).
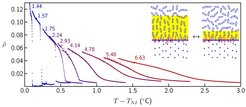
Fig. 4: Temperature dependence of the ellipticity coefficient ρ of a surfactant-laden liquid-crystal/water interface in the temperature range above the nematic - isotropic transition temperature TNI; ρ is a linear measure of the thickness of the nematic layer at the interface. The curves are obtained with different surfactant concentrations (the number at each curve gives the molar fraction in 10-3) in the liquid crystal volume phase. The discontinuous jump observed for low concentrations indicates a first-order prewetting transition at which the thickness of the nematic wetting layer changes abruptly (see schematic drawings in which the nematic layer is marked in yellow). With increasing surfactant concentration, the prewetting transition is driven towards a critical point beyond which the wetting layer thickness varies continuously.
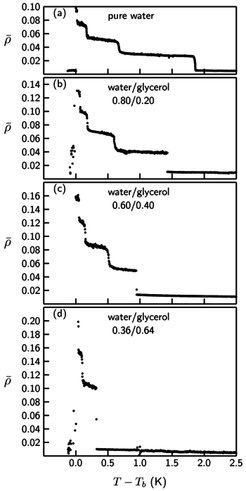
Fig. 5: Influence of a variation of the glycerol content on smectic layering transitions. The figures show the temperature dependence of the ellipticity coefficient ρ of the interface of 12CB (doped with monoolein, mole fraction xs = 0.016) to aqueous phases with different glycerol content; the volume ratio between water and glycerol is indicated in each panel. Tb denotes the bulk smectic-A - isotropic transition temperature of the sample. The stepwise increase of ρ with decreasing temperature indicates the successive formation of molecular smectic layer. With increasing glycerol content the layering transitions shift towards the bulk smectic-A - isotropic transition. The same behavior is observed when the bulk concentration of monoolein is decreased.
![Fig. 6: Influence of a variaition of the glycerol content on nematic prewetting transitions. The figures show the temperature dependence of the ellipticity coefficient ρ of the interface of 9CB (doped with monoolein, mole fraction xs = 0.005) to aqueous phases with different glycerol content; the volume ratio between water and glycerol is indicated in each panel. Tb denotes the bulk nematic - isotropic transition temperature of the sample. With increasing glycerol content a first-order prewetting transition emerges [discontinuity of ρ at T - Tb = 0.6 K in (c)]. The same behavior is observed when the bulk concentration of monoolein is decreased.](/3205521/original-1518437628.jpg?t=eyJ3aWR0aCI6MjQ2LCJvYmpfaWQiOjMyMDU1MjF9--73f07a3e0e5d741b2524bfaab58308feb5dd2d30)
Fig. 6: Influence of a variaition of the glycerol content on nematic prewetting transitions. The figures show the temperature dependence of the ellipticity coefficient ρ of the interface of 9CB (doped with monoolein, mole fraction xs = 0.005) to aqueous phases with different glycerol content; the volume ratio between water and glycerol is indicated in each panel. Tb denotes the bulk nematic - isotropic transition temperature of the sample. With increasing glycerol content a first-order prewetting transition emerges [discontinuity of ρ at T - Tb = 0.6 K in (c)]. The same behavior is observed when the bulk concentration of monoolein is decreased.
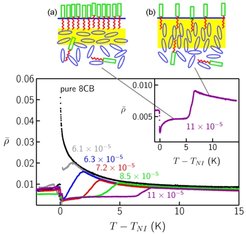
Fig. 7: Temperature dependence of the ellipticity coefficient ρ of the free surface of 8CB doped with different amounts of H18F12; TNI designates the bulk nematic - isotropic transition temperature of 8CB. The mole fraction of H18F12, xHF, is indicated at each curve. The inset shows the data for xHF = 1.1 x 10-4 with an expanded y-scale; note the decrease of ρ as TNI is approached from above. The schematic drawings on top illustrate the different proposed surface structures: dense H18F12 film with a planar aligned nematic layer on an isotropic bulk phase (a), and dilute H18F12 film with a homeotropic aligned nematic layer on an isotropic bulk phase (b).

Fig. 8: Temperature dependence of the ellipticity coefficient ρ of the free surface of 12CB samples doped with different amounts of H18F12; TAI designates the bulk smectic-A - isotropic transition temperature of 12CB. The mole fraction of H18F12, xHF, ranges from 0 (pure 12CB) to 1.1 x 10-3 and is indicated at each curve in units of 10-3.
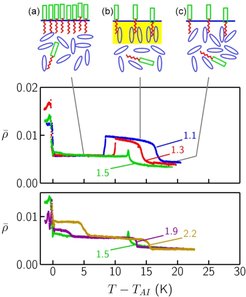
Fig. 9: Temperature dependence of the ellipticity coefficient ρ of the free surface of 12CB samples doped with different amounts of H18F12; TAI designates the bulk smectic-A - isotropic transition temperature of 8CB. The mole fraction of H18F12, xHF, ranges from 1.1 x 10-3 to 1.5 x 10-3 (upper panel) and from 1.5 x 10-3 to 2.2 x 10-3 (lower panel) and is indicated at each curve in units of 10-3. The schematic drawings on top illustrate the different proposed surface structures: dense H18F12 film on an isotropic bulk phase (a), dilute H18F12 film and one smectic layer on an isotropic bulk phase (b), dilute H18F12 film on an isotropic bulk phase (c).

Fig. 10: AFM height images of the surface of droplets of 12CB (a) and 8CB (b) doped with H18F12. The conditions are chosen such that a dense film of the semifluorinated alkane forms on the surface of the liquid crystals. The images demonstrate the presence of surface micelles (lateral diameter approximately 40 nm) which arrange themselves in a dense hexagonal packing. The inset in (a) shows a FFT plot of the height image. Arrows in (b) indicate three terrace steps with a height difference of 1 nm, the blue line indicates the location of the cross section shown in the following figure. The area of each image corresponds to 0.5 x 0.5 µm2.
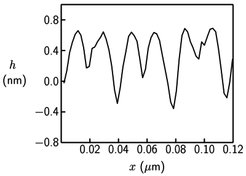
Fig. 11: Cross section through three surface micelles as obtained from the AFM height image shown in Figure 10b (blue line).
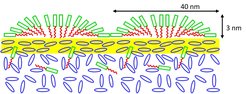
Fig. 12: Schematic of two surface micelles formed by the semifluorinated alkane molecules on the surface of an isotropic liquid crystal. Above the bulk transition to the nematic phase, a thin nematic film with planar anchoring is present.





![Fig. 6: Influence of a variaition of the glycerol content on nematic prewetting transitions. The figures show the temperature dependence of the ellipticity coefficient ρ of the interface of 9CB (doped with monoolein, mole fraction xs = 0.005) to aqueous phases with different glycerol content; the volume ratio between water and glycerol is indicated in each panel. Tb denotes the bulk nematic - isotropic transition temperature of the sample. With increasing glycerol content a first-order prewetting transition emerges [discontinuity of ρ at T - Tb = 0.6 K in (c)]. The same behavior is observed when the bulk concentration of monoolein is decreased. Fig. 6: Influence of a variaition of the glycerol content on nematic prewetting transitions. The figures show the temperature dependence of the ellipticity coefficient ρ of the interface of 9CB (doped with monoolein, mole fraction xs = 0.005) to aqueous phases with different glycerol content; the volume ratio between water and glycerol is indicated in each panel. Tb denotes the bulk nematic - isotropic transition temperature of the sample. With increasing glycerol content a first-order prewetting transition emerges [discontinuity of ρ at T - Tb = 0.6 K in (c)]. The same behavior is observed when the bulk concentration of monoolein is decreased.](/3205521/original-1518437628.jpg?t=eyJ3aWR0aCI6ODQ4LCJmaWxlX2V4dGVuc2lvbiI6ImpwZyIsIm9ial9pZCI6MzIwNTUyMX0%3D--cac6995fdc1ca3c483320c2af81e2680168398d0)





