Convection - Experiments
Two-phase convection
Droplet condensation in the wake of cold drops falling through a warm saturated atmosphere
In order to investigate processes relevant for cloud formation, we also perform moist turbulent RBC experiments in a two-phase binary gas mixture composed out of SF6 and He at Ra numbers of up to 108 in a small Γ = 3.0 cell. Here, helium mimics the dry component of the atmosphere (i.e., nitrogen), while SF6 mimics the water that undergoes phase transitions from a liquid to the vapour. As the top plate temperature is colder and the bottom warmer than the saturation temperature, SF6 condenses at the top plate, forming cold liquid drops that drip down to the bottom.
We found that such a cold drop falling through an SF6-saturated atmosphere causes the nucleation of new liquid droplets in its wake (see fig. 1). The droplet formation is due to homogeneous nucleation as cold saturated vapor from the drop mixes with warm saturated vapor of its environment, producing supersaturated vapor above the critical saturation ratio necessary. As the amount of liquid in the cell was reduced such that no liquid pool formed above the bottom plate, a layer of small droplets was observed in the cell, very similar to an atmospheric cloud layer (see fig. 2). The layer marked a border between a sub-saturated region in the lower part of the cell and a supersaturated region in the upper part. Small micro-droplets that slowly settle due to gravity exist in the upper part but start evaporating as soon as they reach the lower part of the cell.
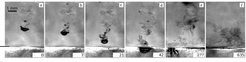
A large cold liquid SF6 drop falls through a saturated vapor atmosphere. Due to mixing of warm and cold saturated vapors in the wake of the drop, the saturation increases above its critical value and small droplets condense due to homogeneous nucleation. The short black line at the left of (a) marks the liquid surface at the bottom.
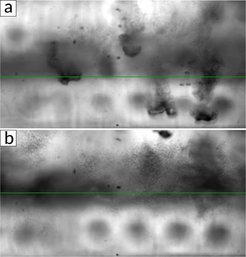
Figure 2:
Snapshots of clouds in a SF6-Helium binary mixture at two different times. Cold drops are falling from the top plate (a) that cause droplet nucleation in their wake (visible in b). These micro-droplets are only stable in the supersaturated region (above the green line) but evaporate in the sub-saturated region (below the green line)

