Mechanics and dynamics of biological adhesion
Cell-nanoparticle interaction: adhesion, motility and internalization dynamics
A first step in investigating the interaction between nanomaterials and cells should be to define their potential risks in a dose-response relationship employing in vitro cell cultures, before addressing more subtle or desired effects at lower concentrations and possibly in vivo. Therefore, plenty of classical, established biochemical viability assays are available and frequently used, like the assessment of metabolic activity, membrane penetration, activation of reactive oxygen species or apoptotic/necrotic pathways stimulation.
The comparison to lab on a chip approaches employing interface sensitive sensors however shows their superior sensititvity especially when monitoring viscoelasticity of cells (7, 4) or passive electrical properties like the trancellular resistance (3, 4), but also active processes like adhesion to (6), micromotility on and shape-, material- or functionalization-dependent (1-3, 5, 8) internalization of nanoparticles.
Over the past years and in the framework of national fundings and collaborations between departments from organic and physical chemistry as well as physics and medicine, we have addressed the adhesion, migration and internalization dynamics of mainly epithelial cells employing novel interface biosensors to polymeric nanoshells (1), MnO and Fe2O3 nanoparticles (2), gold-nanorods or -spheres (3, 4, 5) and quantum dots (3, 8) as well as aggregates and thereby rated possible risks.
We recently also started to address nanoparticle uptake and internalization dynamics of nanoparticles employing Dictyostelium.
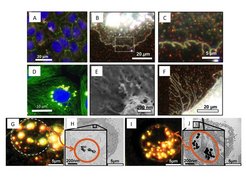
Various cell-interaction stages with the following nanoparticles: CTAB or PEG-coated gold-nanorods in confluent epithelial cell layer imaged in dark-field microscopy (B, C) and combined with epifluorescence (A), with SEM imaging (E-F) or TEM imaging of thin cell segments (G, H: gold-rods; I, J gold-spheres). In D: Quantum-dot aggregates in nuclear vicinity withinin an epithelial cell (co-staining of DAPI and microtubules). All images taken from 1-8.
